In 2020 an estimated 9.9 million people fell ill with TB, and 1.5 million people died from TB.
The problem
Africa is facing an increasing burden of drug-resistant tuberculosis setting back tuberculosis control and elimination strategies
Tuberculosis (TB) caused by the bacterium Mycobacterium tuberculosis (M.tb), remains the deadliest infectious disease globally. According to the World Health Organization (WHO) global TB report, the burden of multidrug-resistant TB (MDR-TB); i.e., resistance to at least rifampicin and isoniazid, has increased by an annual rate of over 20% during the period 2009-2017. In 2019, the global burden of Rifampicin-Resistant TB (RR-TB) was estimated at 465,000 cases, 78% of which was MDR-TB. Sub-Saharan Africa carries 25% of the global burden of drug-resistant TB (DR-TB) .Ongoing transmission of DR-TB remains a major obstacle to the United Nations (UN) End-TB strategy. To reach targets of 90% reduction in tuberculosis (TB) incidence and mortality by 2035, implementation of new and improved diagnostic and treatment approaches are urgently warranted. This study will adopt a Triage-and-Treat approach using novel TB diagnostic technologies to guide implementation of short, all-oral regimens for DR-TB.
Our strategy
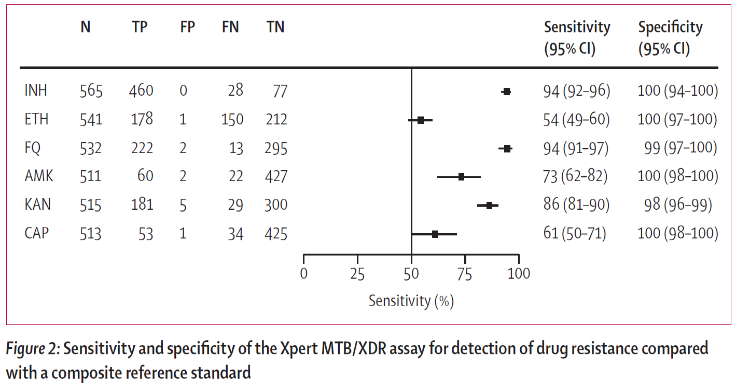
To promptly identify varying TB drug resistance profiles using the new Cepheid Xpert® MTB/XDR assayThe Xpert MTB/XDR cartridge, is a new diagnostic that is being evaluated in this study, intended for use as a reflex test. The assay includes eight genes and promoter regions in M. tb allowing for the detection of resistance to isoniazid, aminoglycosides and fluoroquinolones, ethionamide and the second-line injectables, enabling the diagnosis of pre-XDR-TB in combination with Xpert MTB/RIF or Ultra. |
 |
 |
|
Xpert MTB/XDR Diagnostic Accuracy in Smear Negative CulturesIn this study by 24% of participants had a smear negative, culture positive specimen. The sensitivity and specificity of Xpert MTB/XDR for sputum vs isolates did not differ. Frequency of non-determinate Xpert MTB/XDR results was higher for smear-negative samples vs or smear-positive samples (4% vs 2%) , no notable differences were identified in performance for resistance detection based on smear status. The Xpert MTB/XDR assay performed directly on smear-positive and smear-negative sputum specimens demonstrated high diagnostic accuracy for the detection of resistance to isoniazid, fluoroquinolone amikacin, kanamycin, and capreomycin |
|
The focus of this protocol is to utilise the Xpert MTB/XDR assay to triage and identify TB drug resistance in order to guide the selection of appropriate treatment regimens. With optimal implementation, this new test should improve the selection of appropriate regimens, reducing time to appropriate treatment and improving patient outcomes. Given the recent updated definition, the Xpert XDR assay will diagnose pre-XDR-TB. Additional DST to BDQ and linezolid (LZD) is required to define XDR-TB. |
|
Triage
The study will triage patients onto the most appropriate all oral DR-TB regimen based on their resistance profile.
This approach allows for the rapid identification of patients who have resistance to be triaged to the standard short course regimen, BDQ based all-oral regimens indicated for use in HR-TB, RR-TB and MDR-TB or novel regimens based on the latest available evidence by the World Health Organization. Many of these new regimens have been shown to be effective in clinical trials with common desirable features: good tolerability, all-oral and with shorter treatment duration.

Public Health Impact
Novel diagnostics and short all oral TB drugs used in this study could significantly improve TB treatment outcomes
Given the delays in DR-TB diagnosis, initiation of appropriate treatment, toxicity of drugs in use, identifying novel strategies to improve DR-TB outcomes are important to the success of TB programmes globally. Additionally, a rapid diagnosis coupled with initiation of appropriate treatment could potentially reduce the number of participants that are lost in the care cascade. Generating evidence through implementation research on the feasibility, acceptability and cost effectiveness of novel diagnostics in improving DR-TB outcomes will fast-track translation of these study findings into policy and practice for maximum public health benefit. Given the development of the novel tests and the importance the public health approach in triaging DR-TB participants to suitable all-oral regimens, a study evaluating the strategy is warranted.



